Our first Science experiment that we did was the bouncy egg experiment.
We placed a raw egg (you can do it with a hard boiled egg too) in a jar and covered it with vinegar. We noticed that there were bubbles forming on the outside of the eggshell. We left it in the jar for 24 hours.
We placed a raw egg (you can do it with a hard boiled egg too) in a jar and covered it with vinegar. We noticed that there were bubbles forming on the outside of the eggshell. We left it in the jar for 24 hours.
After 24 hours we took the egg out of the jar. We could see brown foam at the top of the vinegar that was still in the jar. We washed the egg and passed it around. We found that the eggshell had changed and was now rubbery and we were able to squeeze the egg in our fingers.
The chemical reaction that has occurred was that the vinegar (acetic acid) had reacted with the eggshell (calcium carbonate).
We talked about the chemical names for and symbols for each of the substances;
Acetic acid = C2H4O2
Calcium Carbonate = CaCO3
The acetic acid C2H4O2 reacts with the Calcium Carbonate CaCO3 to form carbonic acid H2CO3 and calcium acetate Ca(CH3COO)2. Next the carbonic acid breaks down to form carbon dioxide CO2 and water H2O.
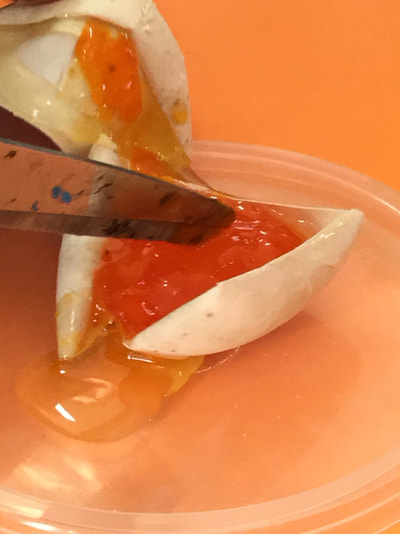
We left the egg out of the jar for 24 hours to see if anything changed. The shell went like paper and felt like paper mache and started to harden. This happens because the eggshell re-absorbs carbon from the carbon dioxide CO2 around us. After 5 days the eggshell became much harder and we cut the egg open to see what it was like inside.
The egg white and yolk had gone like jelly and looked a bit like jam.
The chemical reaction that has occurred was that the vinegar (acetic acid) had reacted with the eggshell (calcium carbonate).
We talked about the chemical names for and symbols for each of the substances;
Acetic acid = C2H4O2
Calcium Carbonate = CaCO3
The acetic acid C2H4O2 reacts with the Calcium Carbonate CaCO3 to form carbonic acid H2CO3 and calcium acetate Ca(CH3COO)2. Next the carbonic acid breaks down to form carbon dioxide CO2 and water H2O.
We left the egg out of the jar for 24 hours to see if anything changed. The shell went like paper and felt like paper mache and started to harden. This happens because the eggshell re-absorbs carbon from the carbon dioxide CO2 around us. After 5 days the eggshell became much harder and we cut the egg open to see what it was like inside.
The egg white and yolk had gone like jelly and looked a bit like jam.
We placed a second egg in the vinegar jar but this time we left it for three days. The shell still reacted the same but this time we could see through the egg and see the yolk.
With this egg we wanted to test how high we could drop it from before it smashed and what it would look like inside. Max and Lucas were in charge of measurements, Josh and Aadit were in charge of recording results and Katelyn was in charge of recording events in photos.
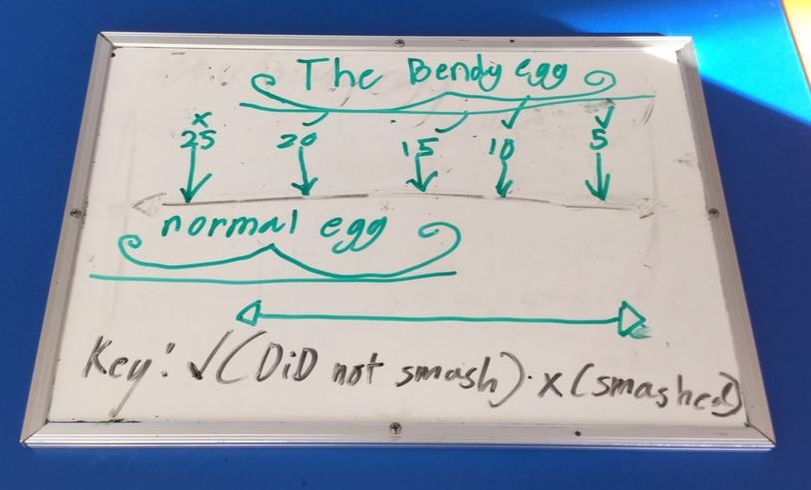
Before we started we predicted what height it would smash at and the majority of us thought it would be between 15cm and 20cm.
With this egg we wanted to test how high we could drop it from before it smashed and what it would look like inside. Max and Lucas were in charge of measurements, Josh and Aadit were in charge of recording results and Katelyn was in charge of recording events in photos.
Before we started we predicted what height it would smash at and the majority of us thought it would be between 15cm and 20cm.
The egg did bounce at different heights but then smashed at 25cm.
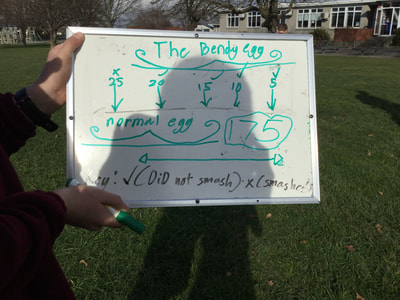
Someone then asked the question "What would happen if it was a normal egg?" so we set the measurements up again and tried with a normal raw egg. We predicted it would smash sooner but we were surprised to find it took until 175cm to smash.
Logan commented that while an eggshell is fragile it still does give protection to the egg.

Our last egg was placed in the vinegar jar for 3 days for the shell to 'dissolve'. Then we placed it in a jar of water and waited to see what would happen.